Entomopathogenic nematodes such as Steinernema carpocapsae and Heterorhabditis bacteriophora have been used to control white grubs that feed turfgrass in your yard. When applied in turf these nematodes search and infect white grubs. They infect grub insects through the natural openings and once inside they release symbiotic bacteria in the body cavity of grub. Bacteria multiply and kill insect within 48 hours of infection.
nematodes
Biological control of filbertworm, Cydia latiferreana with entomopathogenic nematodes /
Filbertworm, Cydia latiferreana is considered as an economically important insect pest of hazelnuts, Corylus avellana in North America. Three entomopathogenic nematode species including Heterorhabditis marelatus Pt. Reyes strain, Steinernema carpocapsae All strain and Steinernema kraussei L137 strain have been tested as biological control agents against filbertworm under both laboratory and field condition (Chambers et al., 2010; Bruck and Walton, 2007). These studies showed that these nematodes can cause about 73–100% mortality of filbertworms (Bruck and Walton, 2007) and can be used to manage overwintering worms on the hazelnut orchard floor (Chambers et al., 2010).
Read following literature for information on the interaction between entomopathogenic nematodes and filbertworm.
Bruck, D.J. and Walton, V.M. 2007. Susceptibility of the filbertworm (Cydia latiferreana, Lepidoptera:Tortricidae) and filbert weevil (Curculio occidentalis, Coleoptera: Curculionidae) to entomopathogenic nematodes. Journal of Invertebrate Pathology. 96: 93–96.
Chambers, U. Bruck, D.J., Olsen, J. and Walton, V.M. 2010. Control of overwintering filbertworm (Lepidoptera: Tortricidae) larvae with Steinernema carpocapsae. Journal of Economic Entomology. 103: 416-422.
Biological control of the red palm weevil, Rhynchophorus ferrugineus with entomopathogenic nematodes /
The red palm weevil, Rhynchophorus ferrugineus is considered as a major pest of palms in the Mediterranean Basin. Because of cryptic habitats of these weevils, their management with chemical insecticides is difficult. It has been demonstrated that the entomopathogenic nematodes have a potential to use as biological control agents against red palm weevils. For example, Steinernema carpocapsae can cause over 80% mortality of weevils under field conditions when applied in a chitosan formulation (Dembilio et al., 2010, Llacer et al., 2009).
Read following literature for more information
Abbas, M.S.T., Saleh, M.M.E. and Akil, A.M. 2001. Laboratory and field evaluation of the pathogenicity of entomopathogenic nematodes to the red palm weevil, Rhynchophorus ferrugineus (Oliv.) (Col.: Curculionidae). Anzeiger Fur Schadlingskunde-Journal of Pest Science. 74: 167-168.
Dembilio, O., Llacer, E., de Altube, M.D.M. and Jacas, J.A. 2010. Field efficacy of imidacloprid and Steinernema carpocapsae in a chitosan formulation against the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Curculionidae) in Phoenix canariensis. Pest Management Science. 66: 365-370.
Llacer, E., de Altube, M.M.M. and Jacas, J.A. 2009. Evaluation of the efficacy of Steinernema carpocapsae in a chitosan formulation against the red palm weevil, Rhynchophorus ferrugineus, in Phoenix canariensis. Biocontrol. 54: 559-565.
Monzer, A.E, and El-Rahman, R.A. 2003. Effect on Heterorhabditis indica of substances occurring in decomposing palm tissues infested by Rhynchophorus ferrugineus. Nematology. 5: 647-652.
Salama, H.S., Abd-Elgawad, M. 2001. Isolation of heterorhabditid nematodes from palm tree planted areas and their implications in the red palm weevil control. Anzeiger Fur Schadlingskunde-Journal of Pest Science. 74: 43-45.
Salama, H.S. and Abd-Elgawad, M. 2002. Activity of heterorhabditid nematodes at high temperature and in combination with cytoplasmic polyhedrosis virus. Anzeiger Fur Schadlingskunde-Journal of Pest Science. 75: 78-80.
Entomopathogenic nematodes can be applied through infected insect host cadavers /
Entomopathogenic nematodes are generally applied as infective juveniles in aqueous suspensions using various techniques including irrigation systems, sprayers and water cans. These nematodes can also be applied through infected host cadavers. It has been demonstrated that the application of nematode infected insect cadavers can provide superior nematode dispersal (Shapiro and Glazer, 1996), infectivity (Shapiro and Lewis, 1999) and survival (Perez et al., 2003) when compared with the nematodes that applied in aqueous suspensions. Please read following literature to learn more about the advantages and disadvantages of applying nematodes through infected insect cadavers.
Creighton, C.S. and Fassuliotis, G. 1985. Heterorhabditis sp. (Nematoda: Heterorhabditidae): a nematode parasite isolated from the banded cucumber beetle Diabrotica balteata. Journal of Nematology. 17: 150–153.
Del Valle, E.E., Dolinksi, C., Barreto, E.L.S. and Souza, R.M. 2009. Effect of cadaver coatings on emergence and infectivity of the entomopathogenic nematode Heterorhabditis baujardi LPP7 (Rhabditida: Heterorhabditidae) and the removal of cadavers by ants. Biological Control 50: 21–24.
Del Valle, E.E., Dolinksi, C., Barreto, E.L.S., Souza, R.M. and Samuels, R.I. 2008. Efficacy of Heterorhabditis baujardi LP77 (Nematoda: Rhabditida) applied in Galleria mellonella (Lepidoptera: Pyralidae) insect cadavers to Conotrachelus psidii (Coleoptera: Curculionidae) larvae. Biocontrol Science and Technology. 18: 33–41.
Perez, E.E., Lewis, E.E and Shapiro-Ilan, D.I. 2003. Impact of host cadaver on survival and infectivity of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under desiccating conditions. Journal of Invertebrate Pathology. 82: 111–118.
Shapiro, D.I and Lewis, E.E. 1999. Comparison of entomopathogenic nematode infectivity from infected hosts versus aqueous suspension. Environmental Entomology. 28: 907–911.
Shapiro, D.I. and Glazer, I. 1996. Comparison of entomopathogenic nematode dispersal from infected hosts versus aqueous suspension. Environmental Entomology. 25: 1455–1461.
Shapiro-Ilan, D.I., Lewis, E.E., Behle, R.W and McGuire, M.R. 2001. Formulation of entomopathogenic nematode-infected-cadavers. Journal of Invertebrate Pathology 78: 17–23.
Shapiro-Ilan, D.I., Lewis, E.E., Tedders, W.L. and Son, Y. 2003. Superior efficacy observed in entomopathogenic nematodes applied in infected-host cadavers compared with application in aqueous suspension, Journal of Invertebrate Pathology 83: 270–272.
Shapiro-Ilan, D.I., Tedders, W.L. and Lewis, E.E., 2008. Application of entomopathogenic nematode-infected cadavers from hard-bodied arthropods for insect suppression. US Patent 7374,773.
Biological control of grape root borer Vitacea polistiformis using entomopathogenic nematodes. /
Efficacy of two entomopathogenic nematodes including Heterorhabditis zealandica strain X1 and H. bacteriophora Strain GPS11 was studied in the field against grape root borer Vitacea polistiformis (Williams et al., 2010). This borer can damage roots of both wild and cultivated Vitis and Muscadinia grapes and is considered as a major pest of grapes grown in the eastern United States. According to Williams et al. (2010), both H. zealandica and H. bacteriophora can cause up to 92% control of grape root borer and they can also persist in the soil for a extended period after their application.
Read following literature for more information on interaction between entomopathogenic nematodes and the grape root borers.
Williams, R.N., Fickle, D.S., Grewal, P.S. and Dutcher, J. 2010. Field efficacy against the grape root borer, Vitacea polistiformis (Lepidoptera: Sesiidae) and persistence of Heterorhabditis zealandica and H. bacteriophora (Nematoda: Heterorhabditidae) in vineyards. Biological Control. 53: 86-91.
Williams, D.S. Fickle, P.S. Grewal and J.R. Meyer. 2002. Assessing the potential of entomopathogenic nematodes to control the grape root borer, Vitacea polistiformis (Lepidopetera: Sesiidae) through laboratory and greenhouse bioassays. Biocontrol Science and Technology 12: 35-42.
Manage insect pests of Strawberries with entomopathogenic nematodes /
Strawberries are one of the most economically grown crops throughout the world and in North America with annual yields ranging from 4-20 tons per acre and average monitory values between $2,800 to $14000 per acre. There are several kinds of insect pests have been reported that attack and cause significant economic losses (over 60%) to this crop. Different species of entomopathogenic have been used as biological control agents against different insect pests of strawberries. It has been demonstrated that the entomopathogenic nematode, Steinernema kraussei can reduce over 81% population of black vine weevil (Ansari et al., 2010; Susurluk and Ehlers, 2008; Willmott et al., 2002). Entomopathogenic nematodes, Heterorhabditis megidis and H. downesi also can reduce 93 and 51% population of black vine weevil, respectively (Boff et al., 2001, 2002; Lola-Luz et al., 2005; Fitters et al., 2001). Populations of black vine weevils were also reduced by application of infective juveniles of Steinernema carpocapsae and S. glaseri (Booth et la., 2002). Steinernema carpocapsae can reduce 51% population of strawberry crown moth (Bruck et al., 2008).
Please read following literature for more information on interaction between insect pests of strawberries and different species entomopathogenic nematodes.
Ansari, M.A., Shah, F.A. and Butt, T.M. 2010. The entomopathogenic nematode Steinernema kraussei and Metarhizium anisopliae work synergistically in controlling overwintering larvae of the black vine weevil, Otiorhynchus sulcatus, in strawberry growbags. Biocontrol Science and Technology. 20: 99-105.
Berry, R.E., Liu, J. and Groth, E. 1997. Efficacy and persistence of Heterorhabditis marelatus (Rhabditida: Heterorhabditidae) against root weevils (Coleoptera: Curculionidae) in strawberry. Environmental Entomology. 26: 465-470.
Boff, M.I.C., van Tol, R.H.W.M. and Smits, P.H. 2002. Behavioural response of Heterorhabditis megidis towards plant roots and insect larvae. Biocontrol. 47: 67-83.
Boff, M.I.C., Wiegers, G.L. and Smits, P.H. 2001. Influence of insect larvae and plant roots on the host-finding behaviour of Heterorhabditis megidis. Biocontrol Science and Technology. 11: 493-504.
Boff, M.I.C., Zoon, F.C. and Smits, P.H. 2001. Orientation of Heterorhabditis megidis to insect hosts and plant roots in a Y-tube sand olfactometer. Entomologia Experimentalis et Applicata. 98: 329-337.
Booth, S.R., Tanigoshi, L.K., Shanks, C.H. 2002. Evaluation of entomopathogenic nematodes to manage root weevil larvae in Washington state cranberry, strawberry, and red raspberry. Environmental Entomology. 31: 895-902.
Bruck, D.J., Edwards, D.L. and Donahue, K.M. 2008. Susceptibility of the strawberry crown moth (Lepidoptera : Sesiidae) to entomopathogenic nematodes. Journal of Economic Entomology. 101: 251-255.
Curran, J. 1992. Influence of application method and pest population-size on the field efficacy of entomopathogenic nematodes. Journal of Nematology. 24: 631-636.
Fitters, P.F.L., Dunne, R. and Griffin, C.T. 2001. Vine weevil control in Ireland with entomopathogenic nematodes: optimal time of application. Irish Journal of Agricultural and Food Research. 40: 199-213.
KakouliDuarte, T., Labuschagne, L. and Hague, N.G.M. 1997. Biological control of the black vine weevil, Otiorhynchus sulcatus (Coleoptera: Curculionidae) with entomopathogenic nematodes (Nematoda: Rhabditida). Annals of Applied Biology. 131: 11-27.
Lola-Luz, T. and Downes, M. 2007. Biological control of black vine weevil Otiorhynchus sulcatus in Ireland using Heterorhabditis megidis. Biological Control. 40: 314-319.
Lola-Luz, T., Downes, M. and Dunne, R. 2005. Control of black vine weevil larvae Otiorhynchus sulcatus (Fabricius) (Coleoptera : Curculionidae) in grow bags outdoors with nematodes. Agricultural and Forest Entomology. 7: 121-126.
Simser, D. and Roberts, S. 1994. Suppression of strawberry root weevil, Otiorhynchus-ovatus, in cranberries by entomopathogenic nematodes (Nematoda, Steinernematidae and Heterorhabditidae). Nematologica. 40: 456-462.
Susurluk, A. and Ehlers, R.U. 2008. Sustainable control of black vine weevil larvae, Otiorhynchus sulcatus (Coleoptera: Curculionidae) with Heterorhabditis bacteriophora in strawberry. Biocontrol Science and Technology. 18: 635-640.
Vainio, A. and Hokkanen, H.M.T. 1993. The potential of entomopathogenic fungi and nematodes against Otiorhynchus-ovatus L and O. dubius strom (Col, Curculionidae) in the field. Journal of Applied Entomology-Zeitschrift fur Angewandte Entomologie. 115: 379-387.
Willmott, D.M., Hart, A.J., Long, S.J., Edmondson, R.N. and Richardson, P.N. 2002. Use of a cold-active entomopathogenic nematode Steinernema kraussei to control overwintering larvae of the black vine weevil Otiorhynchus sulcatus (Coleoptera: Curculionidae) in outdoor strawberry plants. Nematology. 4: 925-932.
Wilson, M., Nitzsche, P. and Shearer, P.W. 1999. Entomopathogenic nematodes to control black vine weevil (Coleoptera : Curculionidae) on strawberry. Journal of Economic Entomology. 92: 651-657.
Entomopathogenic Nematode Facts /
Entomopathogenic nematodes (EPNs) of the two genera Steinernema Travassos, 1927 and Heterorhabditis Poinar, 1976 in the order Rhabdita kill most insects but they are harmless to some beneficial insects (e.g. honey bees), higher animals and environment.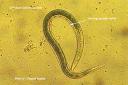 Third-stage juvenile is the only free-living stage in the life cycle of the nematode known as the infective juvenile or dauer juvenile that found in soil and can seek, infect and kill their insect hosts.
Third-stage juvenile is the only free-living stage in the life cycle of the nematode known as the infective juvenile or dauer juvenile that found in soil and can seek, infect and kill their insect hosts.
These infective juveniles are mutualistically associated with symbiotic bacteria (Xenorhabdus spp. or Photorhabdus spp.) in the family Enterobacteriaceae, which are capable of causing disease in insect pests and killing them.
Species of genus, Xenorhabdus are specifically assocaited with the members of the nematode genusSteinernema and Photorhabdus species are associated with the members of nematode genusHeterorhabditis.
In this mutualistic relationship, the nematode infective juveniles provides protection for bacterium outside the insect host (as bacterium is unable to survive in the outside environment i.e. soil or water) and a means of transmission to new hosts and in return bacteria provides nutrients required for the nematode development and reproduction.
Infective juveniles are adapted to remain in the soil environment without feeding until they find a suitable host.
They are also resistant to unfavorable environmental conditions such as desiccation, heat and freezing.
EPNs can infect soil dwelling stages of butterflies, moths, beetles, flies, crickets and grasshoppers.
Infective juveniles of different nematode species employ different foraging strategy to find and infect their insect hosts. For example, Heterorhabditis bacteriophora is a cruiser forager meaning that it actively finds out or hunts its prey, Steinernema carpocapsae is an ambusher forager that sits and wait for a pray to pass by and S. feltiae and S. rivobrave are intermediate foragers.
EPNs are now commercially produced using both in vivo (in living host) and in vitro (in artificial medium) techniques.
Since EPNs have a wide host range, they are currently used as potential biological control agents to manage insect pests of many field crops, greenhouse and nursery plants, horticultural crops, turfgrass, and in some instances insect pests of animals and humans.
EPNs also have a potential to use as biocontrol agents against plant-parasitic nematodes.
Commercially produced nematode infective juveniles can be stored for extended periods and easily applied in aqueous suspensions in the field using traditional sprayers.
Also, EPNs are compatible with several chemical fungicides, insecticides, nematicides and herbicides, and therefore, they can be easily included in IPM programs.
Under current pesticide regulations, the U.S. Environmental Protection Agency has exempted these biological control agents from registration.