The Japanese beetle, Popillia japonica, is a most economically important pest of many ornamental plants and turf grasses. Larvae of these beetles are called white grubs that generally feed on roots of over 300 plants but their primary food source is grass roots.
Read MoreBeneficial nematodes
Kill slugs and snails with parasitic nematode, Phasmarhabditis hermaprodita /
Biological control of slugs and snails with parasitic nematode, Phasmarhabditis hermaprodita
-
Slugs (Mollusca: Gastropoda) are considered as important pests of many agricultural and horticultural crops throughout the world.
-
Recently, a slug parasitic nematode, P. hermaprodita has been commercialized as a biological molluscicide by MicroBio Ltd, UK and sold under the trade name "Nemaslug".
-
Phasmarhabditis hermaprodita as been found to be associated with several different bacteria rather than one particular species but the association with a bacterium, Moraxella oslensis proved to be highly pathogenic to gray garden slug (Deroceras reticulatum) and preferred bacterium for mass production of this nematode in monoxenic culture.
-
Like entomopathogenic nematodes, slug parasitic nematode infective juveniles or dauer juveniles move through soil, locate slugs and infect. They penetrate slugs through a natural opening at the backside of the mantle. Once inside, the dauer juveniles release bacterial cells, start feeding on multiplying bacteria and develop into self-fertilizing hermaphrodites. Nematode- bacteria complex can cause the death of the slug within 7-21 days after infection.
-
Phasmarhabditis hermaprodita can attack and kill several species of slugs including Arion ater, A. intermedius, A. distinctus, A. silvaticus, D. reticulatum, D. caruanae, Tandonia budapestensis and T. sowerbyi.
-
Phasmarhabditis hermaprodita can also parasitize several species of snails including Cernuella virgata, Cochlicella acuta, Helis aspersa, Monacha cantiana, Lymnaea stagnalis and Theba pisana.
-
It has been demonstrated that slug parasitic nematodes when applied at the rate of 3x 109 infective juveniles/hectare can give better control of slugs than standard chemical molluscicide, Methiocarb pellets.
-
For more information on insect and slug parasitic nematodes read a book "Nematodes As Biocontrol Agents" by Grewal, P.S. Ehlers, R.-U., Shapiro-Ilan, D. (eds.). CAB publishing, CAB International, Oxon.
Entomopathogenic Nematodes are considered as excellent biocontrol agents /
Why do entomopathogenic nematodes are considered as excellent biocontrol agents? Because they......
- have a broad host range.
- have the ability to search actively for hosts.
- have the ability to kill their hosts rapidly within 24-48 hours.
- have the potential to recycle in the soil environment.
- have no deleterious effects on humans, other vertebrate animals, non-target organisms and plants.
- have no negative effects on environment.
- can be easily mass produced using both in vivo and in vitro methods.
- can be easily applied using traditional insecticide spraying equipments.
- are compatible with many chemical insecticides and biopesticides.
- have been exempted from registration and regulation requirement by US Environmental Protection Agency (EPA) and similar agencies in many other countries.
Why entomopathogenic nematodes are safe to use as biological control agents against insect pests? /
Because....... 1. Entomopathogenic nematodes and their symbiotic bacterium have no detrimental effects on animals and plants. 2. Both nematodes and their symbiotic bacteria do not cause any harm to the personnel involved in their production and application. 3. Entomopathogenic nematode treated agriculture products are safe to handle and eat. 4. Entomopathogenic nematodes and symbiotic bacteria do not have any pathogenic effects on humans or animals. 5. When applied in the soil, entomopathogenic nematodes have also no negative effect on beneficial nematodes (bacteriovore, fungivore, omnivore and predatory) and other microbial communities. 6. Finally, entomopathogenic nematodes are non-polluting and thus environmentally safe.
How entomopathogenic nematodes find their insect hosts (Foraging Strategies) /
Infective juveniles of entomopathogenic nematodes use three different strategies to find their insect hosts.1. Ambush foraging: Ambushers such as Steinernema carpocapsae and S. scapterisci have adapted "sit and wait" strategy to attack highly mobile insects (billbugs, sod webworms, cutworms, mole-crickets and armyworms) when they come in contact at the surface of the soil. These nematodes do not respond to host released cues but infective juveniles of some Steinernema spp can stand on their tails (nictate) and easily infect passing insect hosts by jumping on them. Since highly mobile insects live in the upper soil or thatch layer, ambushers are generally effective in infecting more insects on the surface than deep in the soil. 2. Cruise foraging: Cruiser nematodes such as Heterorhabditis bacteriophora, H. megidis, Steinernema glaseri and S. kraussei generally move actively in search of hosts and therefore, they are distributed throughout the soil profile and more effective against less mobile hosts such as white grubs and black vine weevils. Cruisers never nictate but respond to carbon dioxide released by insects as cues. 3. Intermediate foraging: Some nematode species such as Steinernema feltiae and S.riobrave have adapted a strategy in between ambush and cruise strategies called an intermediate strategy to attack both the mobile and sedentary/less mobile insects at the surface or deep in the soil. Steinernema feltiae is highly effective against fungus gnats and mushroom flies whereas S.riobrave is effective against corn earworms, citrus root weevils and mole crickets.
Symbiotic bacteria of Heterorhabdits nematodes- Photorhabdus species /
- Heterorhabditis amazonensis- undescribed
- H. argentinensis- P. temperata
- H. bacteriophora- Photorhabdus luminescens subsp. laumondii TT01, P. luminescens kayaii subsp. nov., P. luminescens thracensis subsp. nov., P. temperate
- H. baujardi- undescribed
- H. brevicaudis- P. luminescens
- H. downesi- Photorhabdus sp
- H. floridensis- undescribed
- H. georgiana- undescribed
- H. hambletoni- undescribed
- H. hawaiiensis- P. luminescens
- H. heliothidis- undescribed
- H. hepialius- P. luminescens
- H. hoptha- undescribed
- H. indica- P. luminescens
- H. marelata- P. luminescens
- H. megidis- P. temperata subsp. temperata XlNach
- H. mexicana- undescribed
- H. poinari- Photorhabdus sp
- H. safricana- undescribed
- H. taysearae- undescribed
- H. zealandica- P. temperata
Visit this blog again for new updates (if any) on Heterorhabditis species and their associated bacterial species
Symbiotic bacteria of Steinernematid nematodes- Xenorhabdus species /
- Steinernema abbasi- undescribed
- S. aciari- undescribed
- S. affine-Xenorhabdus bovienii
- S. akhursti- undescribed
- S. anatoliense- undescribed
- S. apuliae- undescribed
- S. arenarium- X. kozodoii
- S. ashiuense- undescribed
- S. asiaticum- undescribed
- S. australe- X. magdalenensis
- S. backanense- undescribed
- S. beddingi- undescribed
- S. bicornutum- X. budapestensis
- S. carpocapsae- X. nematophila
- S. caudatum- undescribed
- S. ceratophorum- undescribed
- S. cholashanense- undescribed
- S. cubanum- X. poinarii
- S. cumgarense- undescribed
- S. diaprepesi- undescribed
- S. eapokense- undescribed
- S. feltiae- X. bovienii
- S. glaseri- X. poinarii
- S. guangdongense- undescribed
- S. hebeiense- undescribed
- S. hermaphroditum- undescribed
- S. intermedium - X. bovienii
- S. jollieti-undescribed
- S. karii- undescribed
- S. khoisanae- undescribed
- S. kraussei- X. bovienii
- S. kushidai- X. japonica
- S. leizhouense- undescribed
- S. litorale- undescribed
- S. loci- undescribed
- S. longicaudum- undescribed
- S. monticolum- undescribed
- S. neocurtillae- undescribed
- S. oregonense- undescribed
- S. pakistanense- undescribed
- S. puertoricense- X. romanii
- S. rarum- X. szentirmaii
- S. riobrave- Xenorhabdus sp
- S. ritteri- Xenorhabdus sp
- S. robustispiculum- undescribed
- S. sangi- undescribed
- S. sasonense- undescribed
- S. scapterisci- X. innexi
- S. scarabaei- X. koppenhoeferi
- S. serratum- X. ehlersii
- S. siamkayai- X. stockiae
- S. sichuanense- X. bovienii
- S. silvaticum- undescribed
- S. tami- Xenorhabdus sp
- S. texanum- undescribed
- S. thanhi- undescribed
- S. thermophilum- X. indica
- S. websteri- undescribed
- S. weiseri- undescribed
- S. yirgalemense- undescribed
Visit this blog again for new updates (if any) on Steinernema species and their associated bacterial species
Beneficial Nematodes: Steinernema and Heterorhabditis species /
Entomopathogenic nematodes in the genera Steinernema and Heterorhabditis are recognized as insect-parasitic nematodes, beneficial nematodes, biocontrol agents, biological control agents, biological insecticides or biopesticides. These nematodes are also recognized as pathogens or microbial control agents because of their symbiotic association with bacteria (Xenorhabdus and Photorhabdus spp.) that are mainly pathogenic to insects. Because of mutualistic relationship with pathogenic bacteria these nematodes are named as entomopathogenic nematodes.
These beneficial nematodes contribute to the regulation of natural populations of insects. However, the population of naturally occurring entomopathogenic nematodes is normally not high enough to manages soil dwelling plant pests. Therefore, during last 3-4 decades, these live nematodes have been commercially mass produced and inundatively applied to control many garden insects, turfgrass insects, nursery insects, greenhouse insects and insects that feed on different field crops.
Use of this natural control of insects is beneficial for both the environment and humans because it reduces use of chemical insecticides/pesticides.
These biopesticides (entomopathogenic nematodes and their symbiotic bacteria) are safe to produce and not harmful to users, application personnel, mammals, most beneficial insects or plants.
Since entomopathogenic nematodes do not cause any health risk to the consumers of nematode treated agricultural produce and damage to the environment, they are exempted from registration requirements in most countries.
These biological control agents have also no detrimental effect on other benefical nematodes including bacterial feeders, some fungal feeders (Aphelenchus sp.), predatory nematodes and other soil microbial communities.
But entomopathogenic nematodes can be detrimental to plant-parasitic nematodes that are responsible for causing a tremendous economic loss to our agriculture industry throughout world. It has been demonstrated that entomopathogenic nematodes can suppress the populations of many economically important plant-parasitic nematodes including foliar nematodes, potato cyst nematodes, ring nematodes, root-knot nematodes, root lesion nematodes, sting nematodes, stubby root nematodes and stunt nematodes.
Symbiotic bacterial genus, Photorhabdus /
known species of symbiotic bacterial genus Photorhabdus associated with a nematode genus Heterorhabditis. Identification based on colony morphology and molecular techniques
- Photorhabdus luminescens (Thomas and Poinar 1979) Boemare et al. 1993
- P. temperata
- P. luminescens subsp. luminescens subsp. nov., Fischer-Le Saux, Viallard, Brunel, Normand & Boemare, 1999
- P. luminescens subsp. akhurstii subsp. nov., Fischer-Le Saux, Viallard, Brunel, Normand & Boemare, 1999
- P. luminescens subsp. kayaii subsp. nov., Hazir, Stackebrandt, Lang, Schumann, Ehlers & Keskin, 2004
- P. luminescens subsp. laumondii subsp. nov., Fischer-Le Saux, Viallard, Brunel, Normand & Boemare, 1999
- P.luminescens subsp. sonorensis, Orozco, Hill & Stock, 2013
- P. temperata sp. nov., Fischer-Le Saux, Viallard, Brunel, Normand & Boemare, 1999
- P. temperata subsp. temperata subsp. nov., Fischer-Le Saux, Viallard, Brunel, Normand & Boemare, 1999
- P. luminescens subsp. thracensis subsp. nov., Hazir, Stackebrandt, Lang, Schumann, Ehlers & Keskin, 2004
Visit this blog again for new updates (if any) on Photorhabdus species
Symbiotic bacterial genus, Xenorhabdus Thomas and Poinar 1979 /
known species of symbiotic bacterial genus Xenorhabdus Thomas and Poinar 1979 associated with a nematode genus Steinernema. Identification based on colony morphology and molecular techniques
- Xenorhabdus beddingii (Akhurst 1986) Akhurst and Boemare 1993
- X. bovienii (Akhurst 1983) Akhurst and Boemare 1993
- X. budapestensis Lengyel, Lang, Fodor, Szállás, Schumann, Stackebrandt, 2005
- X. cabanillasii Tailliez, Pagès, Ginibre & Boemare, 2006
- X. doucetiae Tailliez, Pagès, Ginibre & Boemare, 2006
- X. ehlersii Lengyel, Lang, Fodor, Szállás, Schumann, Stackebrandt, 2005
- X. griffiniae Tailliez, Pagès, Ginibre & Boemare, 2006
- X. hominickii Tailliez, Pagès, Ginibre & Boemare, 2006
- X. indica Somvanshi, Lang, Ganguly, Swiderski, Saxena, & Stackebrandt 2006
- X. innexi Lengyel, Lang, Fodor, Szállás, Schumann, Stackebrandt, 2005
- X. japonica Nishimura et al. 1995
- X. koppenhoeferi Tailliez, Pagès, Ginibre & Boemare, 2006
- X. kozodoii Tailliez, Pagès, Ginibre & Boemare, 2006
- X. magdalenensis, Tailliez, Pages, Edgington, Tymo, & Buddie, 2012
- X. mauleonii Tailliez, Pagès, Ginibre & Boemare, 2006
- X. miraniensis Tailliez, Pagès, Ginibre & Boemare, 2006
- X. nematophila (Poinar and Thomas 1965) Thomas and Poinar 1979
- X. poinarii (Akhurst 1983) Akhurst and Boemare 1993
- X. romanii Tailliez, Pagès, Ginibre & Boemare, 2006
- X. stockiae Tailliez, Pagès, Ginibre & Boemare, 2006
- X. szentirmaii Lengyel, Lang, Fodor, Szállás, Schumann, Stackebrandt, 2005
Visit this blog again for new updates (if any) on Xenorhabdus species
Life cycle of entomopathogenic nematodes (EPNs) /
Entomopathogenic nematode life cycle
- EPNs complete most of their life cycle in insects with an exception of infective juveniles, the only free-living stage found in soil.
- Infective juveniles of both Steinernema and Heterorhabditis locate a host and enter through its natural body openings such as mouth, anus or spiracles.
- Infective juveniles of Heterorhabditis also enter through the intersegmental members of the host cuticle.
- Infective juveniles then actively penetrate through the midgut wall or tracheae into the insect body cavity (hemocoel) containing insect blood (haemolymph).
- Once in the body cavity, infective juvenile releases symbiotic bacteria from its intestine in the insect haemolymph.
- Bacteria start multiplying in the nutrient-rich haemolymph and infective juveniles recover from their arrested state (dauer stage) and start feeding on multiplying bacteria and disintegrated host tissues.
- Toxins produced by the developing nematodes and multiplying bacteria in the body cavity kill the insect host usually within 48 hours.
- These bacteria also produce a plethora of metabolites, toxins and antibiotics with bactericidal, fungicidal and nematicidal properties, which ensures monoxenic conditions for nematode development and reproduction in insect cadaver.
- Heterorhabditid and Steinernematid nematodes differ in their mode of reproduction. For example, in heterorhabditid nematodes, the first generation individuals are produced by self-fertile hermaphrodites (hermaphroditic) but subsequent generation individuals are produced by cross fertilization involving males and females (amphimictic). In Steinernematid nematodes with an exception of one species, all generations are produced by cross fertilization involving males and females (amphimictic).
- Depending on availability of food resource, both heterorhabditid and steinernematid nematodes generally complete 2-3 generations within insect cadaver and emerge as infective juveniles to seek new hosts.
- Generally, life cycle of entomopathogenic nematodes (from infective juvenile penetration to infective juvenile emergence) is completed within 12- 15 days at room temperature. The optimum temperature for growth and reproduction of nematodes is between 25 and 300C.
Species of the genus Heterorhabditis Poinar, 1976 /
Known species of Heterorhabditis Poinar, 1976 with a biocontrol potential- Identification based on morphological and molecular techniques
- Heterorhabditis amazonensis Andalo, Nguyen, & Moino, 2006
- H. argentinensis Stock, 1993
- H. atacamensis, Edgington, Buddie, Moore, France, Merino, & Hunt, 2011
- H. bacteriophora Poinar, 1976
- H. baujardi Phan, Subbotin, Nguyen & Moens, 2003
- H. brevicaudis Liu, 1994
- H. downesi Stock, Griffin & Burnell, 2002
- H. floridensis Nguyen, Gozel, Koppenhofer, & Adams, 2006
- H. georgiana Nguyen, Shapiro-Ilan, & Mbata, 2008
- Heterorhabditis gerrardi, Plichta, Joyce, Clarke, Waterfield, & Stock, 2009
- H. hambletoni (Pereira, 1937) Poinar, 1976
- H. hawaiiensis Gardner, Stock & Kaya, 1994
- H. heliothidis (Khan, Brooks & Hirschman, 1976) Poinar, Thomas & Hess, 1977
- H. hepialius Stock, Strong & Gardner, 1996
- H. hoptha (Turco, 1970), Poinar, 1979
- H. indica Poinar, Karunakar & David, 1992
- H. marelata Liu & Berry, 1996
- H. megidis Poinar, Jackson & Klein, 1988
- H. mexicana Nguyen, Shapiro-Ilan, Stuart, MCCoy, James & Adams, 2004
- H. poinari Kakulia & Mikaia, 1997
- H. safricana Malan, Nguyen, deWaal, & Tiedt, 2008
- Heterorhabditis sonorensis, Stock, Rivera-Orduno, & Flores-Lara, 2009
- H. taysearae Shamseldean, El-Sooud, Abd-Elgawad & Saleh, 1996
- H. zealandica Poinar, 1990
Visit this blog again for new updates (if any) on Heterorhabdtis species
Species of the genus Steinernema Travassos, 1927 /
Known species of Steinernema Travassos, 1927 with a biocontrol potential- Identification was based on morphological and molecular techniques
- Steinernema abbasi Elawad, Ahma & Reid, 1997
- S. aciari Qiu, Yan, Zhou, Nguyen & Pang, 2004
- S. affine (Bovien, 1937) Wouts, Mrácek, Gerdin & Bedding, 1982
- S. akhursti Qiu, Hu, Zhou, Mei, Nguyen, & Pang, 2005
- S. anatoliense Hazir, Stock & Keskin, 2003
- S. apuliae Triggiani, Mracek & Reid, 2004
- S. arenarium (Artyukhovsky, 1967) Wouts, Mrácek, Gerdin & Bedding, 1982
- S. ashiuense Phan, Takemoto & Futai, 2006
- S. asiaticum Shahina, Reid & Rowe, 2002
- S. australe, Edgington, Buddie, Tymo, Hunt, Nguyen, France, Merino, & Moore, 2009
- S. backanense Phan, Spiridonov, Subbotin & Moens, 2006
- S. balochiense Fayyaz, Khanum, Ali, Solangi, Gulsher & Javed, 2015
- S. beddingi Qiu, Hu, Zhou, Pang & Nguyen, 2005
- S. bicornutum Tallosi, Peters & Ehlers 1995
- S. brazilense, Nguyen, Ginarte, Leite, dos Santos, & Harakava, 2010
- S. carpocapsae (Weiser, 1955) Wouts, Mrácek, Gerdin & Bedding, 1982
- S. caudatum Xu, Wang & Li, 1991
- S. ceratophorum Jian, Reid & Hunt 1997
- S. cholashanense Nguyen, Puža & Mrácek, 2008
- S. citrae Stokwe, Malan, Nguyen, Knoetze, & Tiedt, 2011
- S. costaricense Uribe, Mora & Stock, 2007
- S. cubanum Mrá¡cek, Hernandez & Boemare, 1994
- S. cumgarense Phan, Spiridonov, Subbotin & Moens, 2006
- S. dharanaii , Kulkarni, Rizvi, Kumar, Paunikar& Mishra, 2012
- S. diaprepesi Nguyen, & Duncan, 2002
- S. eapokense Phan, Spiridonov, Subbotin & Moens, 2006
- S. fabii Abate, Malan, Tiedt, Wingfield, Slippers, Hurley, 2016.
- S. feltiae (Filipjev, 1934) Wouts, Mrácek, Gerdin & Bedding, 1982
- S. glaseri (Steiner, 1929) Wouts, Mracek, Gerdin & Bedding, 1982
- S. guangdongense Qiu, Fang, Zhou, Pang, & Nguyen, 2004
- S. hebeiense Chen, Li, Yan, Spiridonov & Moens, 2006
- S. hermaphroditum Stock, Griffin, & Chaerani, 2004
- S. innovation Cimen, Lee, Hatting, Hazir, Stock 2015
- S. intermedium (Poinar, 1985) Mamiya, 1988
- S. jeffreyense Malan, Knoetze & Tiedt, 2016
- S. jollieti Spiridonov, Krasomil-Osterfeld & Moens, 2004
- S. karii Waturu, Hunt & Reid, 1997
- S. khoisanae Nguyen, Malan, & Gozel, 2006
- S. kraussei (Steiner, 1923) Travassos, 1927
- S. kushidai Mamiya, 1988
- S. leizhouense Nguyen, Qiu, Zhou, & Pang, 2006
- S. litorale Yoshida, 2004
- S. loci Phan, Nguyen & Moens, 2001
- S. longicaudum Shen & Wang, 1992
- S. monticolum Stock, Choo & Kaya, 1997
- S. neocurtillae Nguyen & Smart, 1992
- S. oregonense Liu & Berry, 1996
- S. pakistanense Shahina, Anis, Reid, Rowe & Maqbool, 2001
- S. papillatum San-Blas, Portillo, Nermut, Puza, & Morales-Montero 2015
- S. phyllophagae Nguyen and Buss, 2011
- S. puertoricense Roman & Figueroa, 1994
- S. puntauvense Uribe, Mora & Stock, 2007
- S. rarum (Doucet, 1986) Mamiya, 1988
- S. riobrave Cabanillas, Poinar & Raulston, 1994
- S. ritteri de Doucet & Doucet, 1992
- S. robustispiculum Phan, Subbotin, Waeyenberge, & Moens, 2005
- S. sangi Phan, Nguyen & Moens, 2001
- S. sasonense Phan, Spiridonov, Subbotin & Moens, 2006
- S. scapterisci Nguyen & Smart, 1992
- S. scarabaei Stock & Koppenhöfer 2003
- S. serratum Liu, 1992
- S. siamkayai Stock, Somsook & Kaya, 1998
- S. sichuanense Mrácek, Nguyen, Tailliez, Boemare & Chen, 2006
- S. silvaticum Sturhan, Spiridonov & Mracek, 2005
- S. tami Luc, Nguyen, Reid & Spiridonov, 2000
- S. texanum Nguyen, Stuart, Andalo, Gozel, & Roger, 2007
- S. thanhi Phan, Nguyen & Moens, 2001
- S. thermophilum Ganguly & Singh, 2000
- S. websteri Cutler & Stock, 2003
- S. weiseri Mrácek, Sturhan & Reid, 2003
- S. xinbinense Ma, Chen, De Clercq, Waeyenberge, Han & Moens, 2012
- S. xueshanense, Mracek, Liu, & Nguyen, 2009
- S. yirgalemense Nguyen, Tesfamariam, Gozel, Gaugler, & Adams, 2005
Visit this blog again for new updates (if any) on Steinernema species
Entomopathogenic Nematode Facts /
Entomopathogenic nematodes (EPNs) of the two genera Steinernema Travassos, 1927 and Heterorhabditis Poinar, 1976 in the order Rhabdita kill most insects but they are harmless to some beneficial insects (e.g. honey bees), higher animals and environment.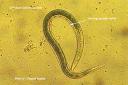 Third-stage juvenile is the only free-living stage in the life cycle of the nematode known as the infective juvenile or dauer juvenile that found in soil and can seek, infect and kill their insect hosts.
Third-stage juvenile is the only free-living stage in the life cycle of the nematode known as the infective juvenile or dauer juvenile that found in soil and can seek, infect and kill their insect hosts.
These infective juveniles are mutualistically associated with symbiotic bacteria (Xenorhabdus spp. or Photorhabdus spp.) in the family Enterobacteriaceae, which are capable of causing disease in insect pests and killing them.
Species of genus, Xenorhabdus are specifically assocaited with the members of the nematode genusSteinernema and Photorhabdus species are associated with the members of nematode genusHeterorhabditis.
In this mutualistic relationship, the nematode infective juveniles provides protection for bacterium outside the insect host (as bacterium is unable to survive in the outside environment i.e. soil or water) and a means of transmission to new hosts and in return bacteria provides nutrients required for the nematode development and reproduction.
Infective juveniles are adapted to remain in the soil environment without feeding until they find a suitable host.
They are also resistant to unfavorable environmental conditions such as desiccation, heat and freezing.
EPNs can infect soil dwelling stages of butterflies, moths, beetles, flies, crickets and grasshoppers.
Infective juveniles of different nematode species employ different foraging strategy to find and infect their insect hosts. For example, Heterorhabditis bacteriophora is a cruiser forager meaning that it actively finds out or hunts its prey, Steinernema carpocapsae is an ambusher forager that sits and wait for a pray to pass by and S. feltiae and S. rivobrave are intermediate foragers.
EPNs are now commercially produced using both in vivo (in living host) and in vitro (in artificial medium) techniques.
Since EPNs have a wide host range, they are currently used as potential biological control agents to manage insect pests of many field crops, greenhouse and nursery plants, horticultural crops, turfgrass, and in some instances insect pests of animals and humans.
EPNs also have a potential to use as biocontrol agents against plant-parasitic nematodes.
Commercially produced nematode infective juveniles can be stored for extended periods and easily applied in aqueous suspensions in the field using traditional sprayers.
Also, EPNs are compatible with several chemical fungicides, insecticides, nematicides and herbicides, and therefore, they can be easily included in IPM programs.
Under current pesticide regulations, the U.S. Environmental Protection Agency has exempted these biological control agents from registration.

